Molar Mass of Hydrated Copper Sulfate
Explanation of how to find the molar mass of CuSO4 5H2O. The percent by mass of water of hydration is calculated as the mass of water of hydration divided by the mass of hydrated copper sulfate.
Solved Sample Data Mass Of Sample Hydrated Copper Ii Chegg Com
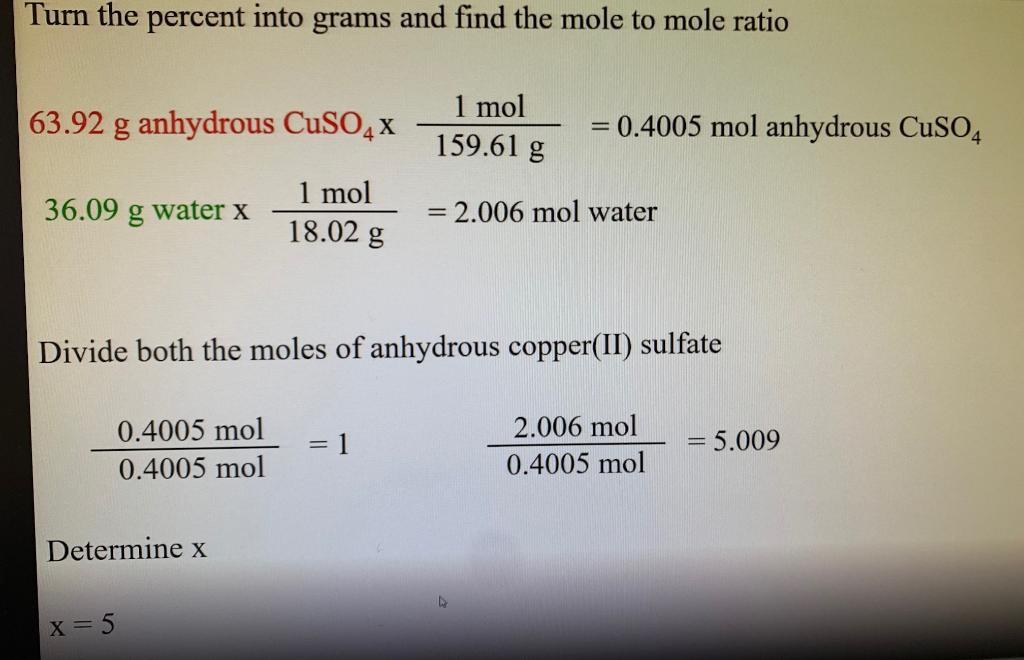
N mMM 353 g 1596 gmol 002212 moles.

. So I did a lab determining the chemical formula of a hydrate. We had to find the molecular formula of a hydrate of copper 2 sulphate CuSO4 xH2O. When considering the chemical properties of this compound the molar mass is 1596 gmol.
Mass of clean dry test tube. The dot in the formula indicates that the two compounds are bound. What is CuSO4 5H2O.
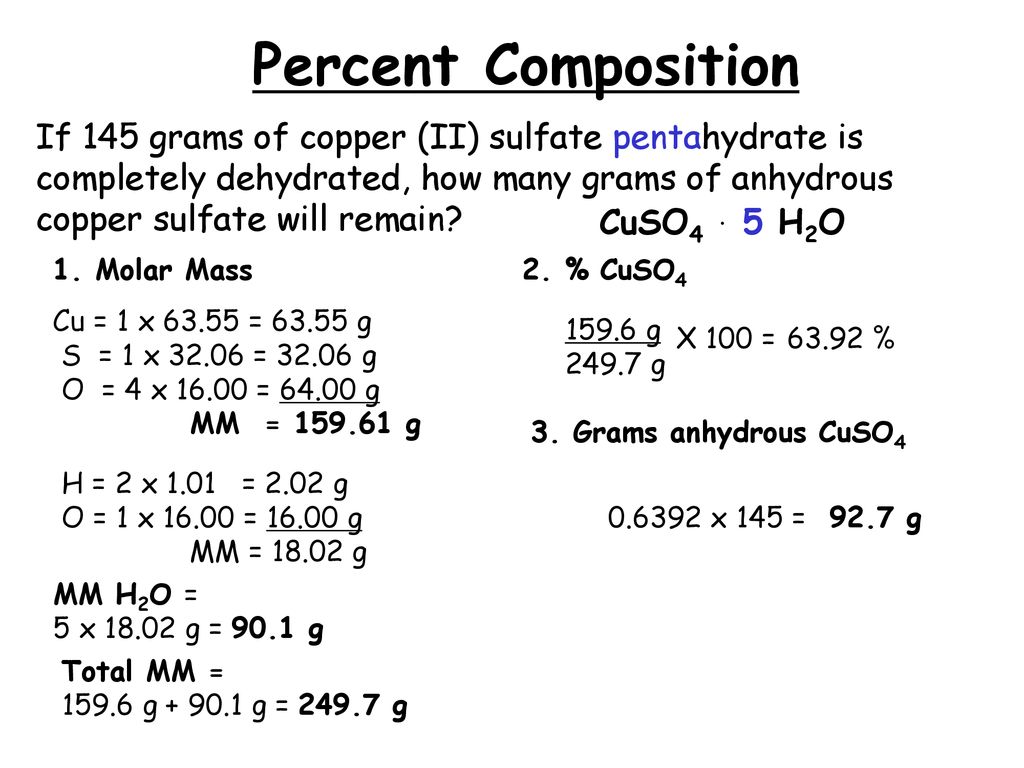
Molar masses were calculated using Molar Mass Calculator and rounded to four significant figures. 2545 g of hydrate is heated and 6385 of the residue is found after heating. So copper II sulfate pentahydrate has a molar mass of.
My observation table looks like this. A sample of copper sulfate pentahydrate CuSO45H2O molar mass of CuSO45H2O 24968 gmol contains 3782g of Cu. Hydrated copper sulfate 1000g anhydrous copper sulfate 591g Per the video the next step is to get the molar mass of the anhydrous copper sulfate and determine the number of moles.
It appears in grey-white colour. Calculate the formula mass. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by.
As the molar mass of copper sulfate pentahydrate is 2497 gmol the amount of moles to start was. 63546 x 1 32066 x 1 159994 x 4 10079 x 10 159994 x 5 2496856 gmol. CuSO4 is composed of Cu 6355 g mol1 S 3206 g mol1 and oxygen 4 15999 g mol1.
How many moles of anhydrous copper sulfate do we have. Cu 63546 S 32066 O 1600 H 1008 Expert Answer. Copper sulfate CuSO4 or CuO4S CID 24462 - structure chemical names physical and chemical properties classification patents literature biological activities.
Calculate the number of moles of water driven off. What is the molar mass of hydrated copper sulfate CuSO4 5H2O. 1 Cu atom and 9 O atoms.
To determine the theoretical by mass use your understanding of molar mass as it pertains to the given formula. Molar mass of CuSO4 1596086 gmol. The dissolution of white anhydrous CuSO4 in water is a truly beautiful chemical reaction.
Calculate the number of moles of anhydrous copperII sulfate formed. N mMM 496 g 2497 gmol 001986 moles. The dot in this formula means.
Copper sulfate is a hydrated crystal with the formula CuSO45H20 and a deep blue color. Copper sulphate CuSO4 Copper has the atomic mass 635 Sulphur 32 Oxygen 16 635 32 164 635 32 64 1595gram - CuSo4 has atomic mass 1595g or approximately 160g 35K views View upvotes Related Answer Keith Earnshaw. 1 formula unit of CuSO45H2O contains.
Is the dehydration and hydration of CuSO4 5h2o reversible. When considering the melting point of copper sulfate it is 110 C and upon further heating the compound decomposes. H1 O16 S32 Cu64 Calculate the mass of water driven off and the mass of anhydrous copperII sulfate formed in your experiment.
A hydrate of copper II sulfate with formula CuSO4xH2O has a molar mass of 2500 gmol. 250 grams What is the formula of hydrated copper sulphate. CuSO4 5H2O is copper II sulfate pentahydrate.
Mass of anhydrous copperII sulfate copied from yesterdays data sheet_____. A Determine the value of x in the formula of the compound b Chem 11. Calculate the molar masses of H 2 O and CuSO 4 Relative atomic masses.
216g Mass of. When its heated the crystals crumble and turn white. The density is 360 gcm3.
Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole. See the answer Given the hydrate of Copper II sulfate as CuSO 4 5H 2 O What is the mole ratio of H 2 O to CuSO 4 give the molar mass of the hydrate Calculate the percentage of H 2 O in the hydrate give the systematic name of the hydrate. So what is its molar mass.
Beautiful macroscopic crystals of CuSO4 5H 2O can be grown whose colour is truly magnificent. The chemical formula of a typical hydrated compound such as copperII sulfate pentahydrate is written as CuSO45H2O. How many grams of oxygen are in this sample.
Water also comes out of the copper sulfate and is able to function as any other type of water would typically function. Mass of hydrated copperII sulfate copied from yesterdays data sheet_____ 2. As the molar mass of anhydrous copper sulfate is 1596 gmol the amount of moles of anhydrous copper sulfate is.
CuSO4 5 H2O Important note. Copper II sulfate pentahydrateA few things to consider when finding the molar mass for CuSO4. When the copper sulfate is heated up it quickly turns to a pure white color that has no hint of blue in it.
4 rows CopperII Sulfate molecular weight. Molar mass of copperii sulfate is 1596086 gmol. What happens when you heat hydrated copper sulfate.
In the formula CuSO 4 x H 2 O x is calculated as the amount of water of hydration divided by the amount of anhydrous copper sulfate. Copper sulfate pentahydrate CuSO45H2O or CuH10O9S CID 24463 - structure chemical names physical and chemical properties classification patents literature. What is the percent water in copperII sulfate pentahydrate CuSO 4 5 H 2 O.
Divide the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiply this fraction by 100. When determining the formula mass for a hydrate the waters of hydration must be included.

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

Percent Composition Practice Problem 3 Cuso4 5h2o Youtube
Molar Mass Molecular Weight Of Cuso4 Copper Ii Sulfate Youtube
Copper Sulfate Pentahydrate Cuso4 5h2o Pubchem

Chapter 10 The Mole The Mole What Do You Ask For When You Buy 2 Shoes 12 Eggs 48 Doughnuts 500 Sheets Of Paper 1 Pair 1 Dozen 4 Dozen 1 Ream Ppt Download

Calculate The Percentage Loss Of Mass Of Hydrated Copper Ii Sulphate Cuso4 5h2o When It Is Completely Dehydrated Cuso4 5h2o Cuso4 5h2o Atomic Weights Are Cu 64 S 32 O 16 H 1

Molar Mass Of Hydrates Youtube

Solved Sample Data Mass Of Sample Hydrated Copper Ii Chegg Com

Molar Mass Molarity Molar Mass Mass In Grams Of One Mole Of An Element Or Compound Numerically Equal To The Atomic Weight Of The Element Or The Sum Ppt Download

General Chemistry Copper Sulfate Lab

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

Calculating The Percent Water In A Hydrate Mr Pauller Youtube

Calculate The Percentage Loss Of Mass Of Hydrated Copper Ii Sulphate Cuso4 5h2o When It Is Completely Dehydrated Cuso4 5h2o Cuso4 5h2o Atomic Weights Are Cu 64 S 32 O 16 H 1

Calculate The Percentage Loss Of Mass Of Hydrated Copper Ii Sulphate Cuso4 5h2o When It Is Completely Dehydrated Cuso4 5h2o Cuso4 5h2o Atomic Weights Are Cu 64 S 32 O 16 H 1

Calculate The Relative Molecular Mass Of Hydrated Copper Sulphate Cuso4 2h2o H 1 O 16 S 32 Cu 64 Experts Please Answer It Urgent Chemistry The Language Of Chemistry 14286463 Meritnation Com

To Find The Formula Of Hydrated Copper Ii Sulfate
Percent Composition In Your Books Pg Ppt Download

E4 Calculations Determination Of A Chemical Formula Copper Chloride Hydrate Youtube
Comments
Post a Comment